
 i_need_contribute
i_need_contribute
Last week, the Food and Drug Administration authorized the Novavax vaccine for distribution as a primary immunization series for adults who have never been vaccinated, rather than a booster. According to the F.D.A., Novavax, a Maryland-based biotechnology company, is expected to finish quality testing “in the next few weeks.”
The C.D.C. panel is expected to meet on Tuesday to discuss who should get the Novavax vaccine.
Novavax hopes its two-dose vaccine will appeal to the nearly 22 percent of Americans who have yet to receive a shot. Instead of utilizing the revolutionary messenger RNA technology used by Pfizer-BioNTech and Moderna, the Novavax vaccine is protein-based — a much older type of vaccine that has been used widely for decades. Skeptics have seized on the rapid development of the Pfizer and Moderna vaccines to undermine the public’s trust in them.
According to C.D.C. data, the average of new doses administered has hovered a little over 200,000 per day, around the lowest level since vaccines became widely available, and tens of millions of people in the United States are still unvaccinated.
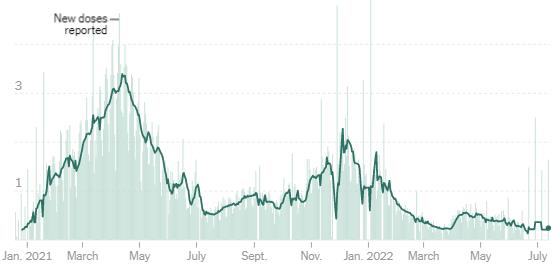
Source: Centers for Disease Control and Prevention | Note: Line shows a seven-day average. The C.D.C., in collaboration with the states, sometimes revises data or reports a single-day large increase in vaccinations from previous dates, which can cause an irregular pattern.
The Biden administration announced last week that the United States would buy 3.2 million Novavax doses, enough to fully vaccinate 1.6 million people. But questions remain about the demand for the new shot. In Europe, only 12.6 million doses of Novavax’s vaccine were distributed since the shot was authorized in December. Europe has distributed more than a billion doses of Pfizer-BioNTech and Moderna vaccines.
Novavax was a little-known biotechnology company before it rose to prominence when Operation Warp Speed, the federal government’s 2020 campaign to develop coronavirus vaccines, made one of its largest deals with the company, agreeing to pay it $1.6 billion.
However, a series of manufacturing delays meant that the shot was not available when the U.S. vaccination campaign began in December 2020, and manufacturing woes have continued to dog the company.