
 i_need_contribute
i_need_contribute


| Country, | Total | New | Total |
| Other | Cases | Cases | Deaths |
| World | 115,287,756 | 365,724 | 2,559,569 |
| USA | 29,370,705 | 56,890 | 529,214 |
| India | 11,139,323 | 15,704 | 157,385 |
| Brazil | 10,647,845 | 58,237 | 257,562 |
| Russia | 4,268,215 | 10,565 | 86,896 |
| UK | 4,188,400 | 6,391 | 123,296 |
| France | 3,783,528 | 22,857 | 87,220 |
| Spain | 3,130,184 | 6,484 | 69,801 |
| Italy | 2,955,434 | 17,083 | 98,288 |
| Turkey | 2,723,316 | 11,837 | 28,706 |
| Germany | 2,462,061 | 6,492 | 71,325 |
| Colombia | 2,259,599 | 4,339 | 59,972 |
| Argentina | 2,118,676 | 6,653 | 52,192 |
| Mexico | 2,089,281 | 2,343 | 186,152 |
| Poland | 1,719,708 | 7,937 | 44,008 |
| Iran | 1,648,174 | 8,495 | 60,267 |
| South Africa | 1,514,815 | 856 | 50,271 |
| Ukraine | 1,357,470 | 5,336 | 26,212 |
| Indonesia | 1,347,026 | 5,712 | 36,518 |
| Peru | 1,338,297 | 5,358 | 46,894 |
| Czechia | 1,252,416 | 12,301 | 20,834 |
| Netherlands | 1,096,433 | 3,981 | 15,649 |
| Canada | 872,747 | 2,714 | 22,045 |
| Chile | 832,512 | 2,742 | 20,684 |
| Romania | 808,040 | 3,950 | 20,509 |
| Portugal | 805,647 | 691 | 16,389 |
| Israel | 785,218 | 5,260 | 5,790 |
| Belgium | 772,294 | 783 | 22,106 |
| Iraq | 703,778 | 4,690 | 13,458 |
| Sweden | 669,113 | 12,882 | |
| Pakistan | 582,528 | 1,163 | 12,938 |
| Philippines | 580,440 | 2,065 | 12,369 |
| Switzerland | 558,622 | 1,130 | 10,005 |
| Bangladesh | 547,316 | 515 | 8,423 |
| Morocco | 484,159 | 393 | 8,645 |
| Serbia | 466,885 | 4,157 | 4,475 |
| Austria | 462,769 | 1,920 | 8,605 |
| Hungary | 435,689 | 2,764 | 15,188 |
| Japan | 433,504 | 731 | 7,933 |
| Jordan | 402,282 | 5,124 | 4,756 |
| UAE | 396,771 | 2,721 | 1,253 |
| Lebanon | 380,036 | 3,098 | 4,805 |
| Saudi Arabia | 378,002 | 302 | 6,505 |
| Panama | 342,019 | 599 | 5,871 |
| Slovakia | 311,002 | 2,077 | 7,388 |
| Malaysia | 304,135 | 1,555 | 1,141 |
| Belarus | 289,136 | 869 | 1,993 |
| Ecuador | 286,725 | 358 | 15,850 |
| Nepal | 274,294 | 78 | 2,777 |
| Georgia | 271,379 | 461 | 3,532 |
| Bulgaria | 252,029 | 2,403 | 10,391 |
| Bolivia | 249,767 | 757 | 11,666 |
| Croatia | 243,458 | 394 | 5,548 |
| Dominican Republic | 240,201 | 203 | 3,118 |
| Azerbaijan | 235,014 | 352 | 3,225 |
| Tunisia | 234,231 | 562 | 8,047 |
| Ireland | 220,630 | 357 | 4,333 |
| Kazakhstan | 214,089 | 658 | 2,540 |
| Denmark | 212,224 | 532 | 2,367 |
| Costa Rica | 205,514 | 428 | 2,820 |
| Lithuania | 199,825 | 426 | 3,263 |
| Greece | 194,582 | 2,312 | 6,557 |
| Kuwait | 193,372 | 1,341 | 1,092 |
| Slovenia | 191,056 | 729 | 3,863 |
| Moldova | 187,847 | 1,400 | 4,002 |
| Palestine | 187,309 | 1,973 | 2,063 |
| Egypt | 183,591 | 581 | 10,778 |
| Guatemala | 175,411 | 758 | 6,412 |
| Armenia | 172,456 | 240 | 3,200 |
| Honduras | 170,985 | 681 | 4,174 |
| Qatar | 164,600 | 463 | 259 |
| Paraguay | 161,530 | 1,082 | 3,218 |
| Ethiopia | 160,813 | 841 | 2,386 |
| Nigeria | 156,496 | 479 | 1,923 |
| Oman | 142,169 | 361 | 1,580 |
| Myanmar | 141,965 | 49 | 3,199 |
| Venezuela | 139,934 | 389 | 1,353 |
| Libya | 134,967 | 840 | 2,216 |
| Bosnia and Herzegovina | 133,088 | 727 | 5,145 |
| Bahrain | 123,531 | 492 | 453 |
| Algeria | 113,430 | 175 | 2,991 |
| Albania | 108,823 | 892 | 1,835 |
| Kenya | 106,470 | 345 | 1,863 |
| North Macedonia | 103,746 | 726 | 3,151 |
| S. Korea | 90,372 | 344 | 1,606 |
| China | 89,923 | 11 | 4,636 |
| Latvia | 87,103 | 645 | 1,638 |
| Kyrgyzstan | 86,308 | 57 | 1,467 |
| Ghana | 84,023 | 607 | |
| Sri Lanka | 83,870 | 318 | 483 |
| Uzbekistan | 79,961 | 35 | 622 |
| Zambia | 79,557 | 555 | 1,104 |
| Montenegro | 76,868 | 585 | 1,023 |
| Norway | 72,234 | 499 | 623 |
| Estonia | 67,739 | 1,113 | 605 |
| El Salvador | 60,491 | 154 | 1,869 |
| Singapore | 59,956 | 8 | 29 |
| Mozambique | 59,914 | 307 | 665 |
| Uruguay | 59,171 | 582 | 617 |
| Finland | 58,645 | 581 | 755 |
| Afghanistan | 55,770 | 11 | 2,446 |
Retrieved from: https://www.worldometers.info/coronavirus/

Paige Blankenship, left, Ohio State University clinic manager, administers one of the first Johnson & Johnson vaccines to Osvaldo Campanella in Columbus, Ohio, on Tuesday.Credit...Jay Laprete/Associated Press
President Biden announced Tuesday that there would be enough doses of the coronavirus vaccine available for the entire adult population in the United States by the end of May, though he said it will take longer to inoculate everyone and he urged people to remain vigilant by wearing masks.
Mr. Biden had previously said there would be enough doses by the end of July. On Tuesday, he said the faster production of the vaccine was in part the result of an agreement by the pharmaceutical giant Merck & Co to help manufacture the new Johnson & Johnson coronavirus vaccine under an unusual deal, brokered by the White House.
He said that agreement, along with other efforts by the government to help Johnson & Johnson produce more doses quickly, will substantially increase the supply of the new vaccine and ramp up the pace of vaccination just as worrisome new variants of the virus have been found in the United States.
“As a consequence of the stepped up process that I ordered, and just outlined, this country will have enough vaccine supply as a target for every adult in America by the end of May,” Mr. Biden said. “By the end of May. That’s progress. Important progress.”
He also said he wanted all teachers vaccinated by the end of this month.
The Merck arrangement, first reported by The Washington Post, comes just days after the Food and Drug Administration granted emergency authorization to the Johnson & Johnson vaccine.
Merck is an experienced vaccine manufacturer whose own attempt at making a coronavirus vaccine was unsuccessful. Mr. Biden described the partnership between the two competitors as “historic,” and said it harks back to his vision of a wartime effort to fight the coronavirus, similar to the manufacturing campaigns waged during World War II.
“This is a type of collaboration between companies we saw in World War II,” the president said.
Both Trump and Biden administration officials had explored enlisting Merck’s help in manufacturing vaccines developed by either Pfizer or Johnson & Johnson. But Jen Psaki, the White House press secretary, said the White House deserved credit for bringing the deal to fruition.
“There’s a difference between conversations and it moving forward,” she said. “I’m only conveying what got it across the finish line.”
Just how quickly Merck will be able to ramp up is unclear. It will take months for the company to convert its facilities to manufacture and package a vaccine that it did not invent, according to two people familiar with Johnson & Johnson’s operations who were not authorized to speak publicly. But one federal official with knowledge of the arrangement, who spoke on the condition of anonymity, said the administration hopes the deal would eventually double the doses that Johnson & Johnson could have manufactured on its own.
Although company executives have promised that the firm will catch up this spring, Johnson & Johnson has been running behind on its manufacturing targets as it tries to ramp up at its new plant in Baltimore. Its initial shipments of doses, delivered this week to states, were manufactured at its plant in the Netherlands.
Under the new agreement, Merck will dedicate two of its facilities to production of the Johnson & Johnson vaccine.
One facility will provide “fill-finish,” the final phase of the manufacturing process during which the vaccine is placed in vials and packaged for shipping. The other will make the “drug substance” — the vaccine itself.
Ms. Psaki said that the federal government will invoke the Defense Production Act to help Merck obtain necessary supplies and equipment, and has also asked the Defense Department to strengthen Johnson & Johnson’s manufacturing effort.
The Johnson & Johnson vaccine is the third to receive emergency authorization from the Food and Drug Administration — following Moderna and Pfizer-BioNTech.
And the pace of the nation’s vaccination effort has been steadily accelerating. As of Monday, about 50.7 million people have received at least one dose of a Covid-19 vaccine, including about 25.5 million people who have been fully vaccinated, according to Centers for Disease Control and Prevention.
While the Pfizer and Moderna vaccines performed somewhat better in clinical trials, all three vaccines are considered safe and effective. And the Johnson & Johnson vaccine has some advantages: It requires only one shot, and studies show it may curb spread of the virus.
The other two vaccines use a new technology called mRNA, which allowed for faster design and testing of vaccine but resulted in a product that required more stringent storage conditions. Johnson & Johnson’s vaccine, which uses viruses to deliver genes into cells, can keep for three months at normal refrigeration temperatures, making it easier to distribute and easier for pharmacies and clinics to stock. At $10 a dose, it is also cheaper than the other two.
Johnson & Johnson’s $1 billion federal contract, signed last year when it had just started developing the vaccine, called for it to deliver 37 million doses by the end of March and a total of 100 million doses by the end of June. The company has now said it can only deliver 20 million doses this month, and senior administration officials have said the vast bulk of those will only be ready in the final weeks of March.
This week, states will receive 3.9 million doses that were manufactured at a Dutch plant and bottled in Grand Rapids, Mich. Johnson & Johnson is expected to mass produce the vaccine at a new Baltimore plant operated by a company called Emergent BioSolutions. Catalent, a different firm, will bottle the doses.
The F.D.A.’s authorization for emergency use, granted late Saturday, covered the Dutch production lines and the Grand Rapids bottling operation. In about two weeks, federal regulators are expected to decide whether to amend that authorization to include the other plants, according to the two people familiar with Johnson and Johnson’s operations. Until then, they said, supply will be uneven and limited.
For nearly a year, Merck has been searching for a way to play a key role in the nation’s vaccination program. Federal officials considered a possible partnership between Merck and Pfizer or Moderna, but Ms. Psaki said the mRNA technology could not be smoothly transferred.
Even with Merck, the two people familiar with Johnson & Johnson’s operations, retooling plants to produce the vaccine will be a laborious, months-long process. By the time it is accomplished the demand for shots may have waned.
https://www.nytimes.com/live/2021/03/02/world/covid-19-coronavirus?name=styln-vaccines-combo®ion=MAIN_CONTENT_2&block=storyline_latest_updates_recirc&action=click&pgtype=Article&impression_id=&variant=1_Show&index=1
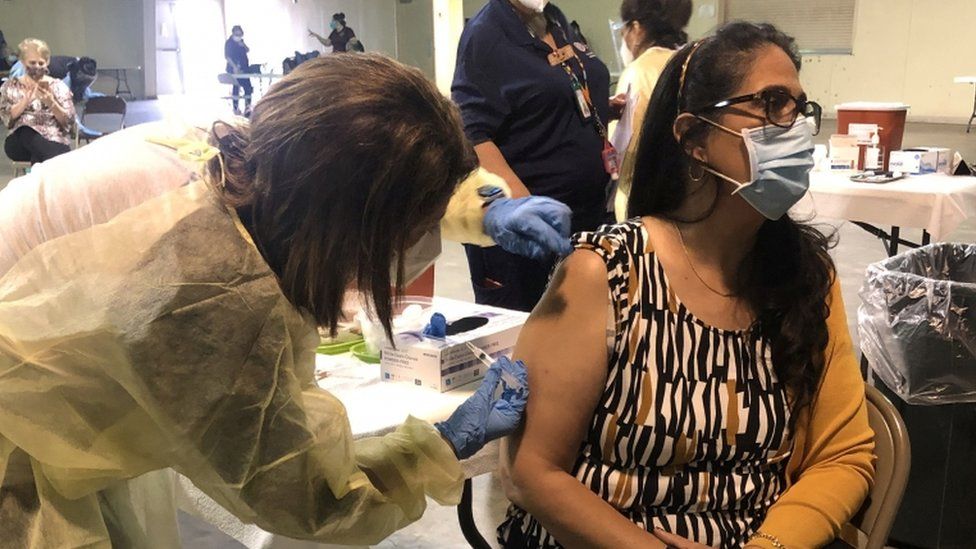
IMAGE COPYRIGHTREUTERS
States across the US have started to ease Covid-19 restrictions, causing concern among health officials
Texas will lift its mask requirement and allow businesses to reopen at full capacity next week, Governor Greg Abbott has announced.
"It is now time to open Texas 100%," the Republican said on Tuesday.
Texas is the largest US state to end its mask mandate. Mr Abbott has faced criticism from his party over the measure, which was imposed last July.
But the administration of US President Joe Biden has made it clear coronavirus restrictions are still necessary.
The announcement in Texas came as similar rules were lifted in other states, including Michigan, Louisiana, and Mississippi, which also ended its mask mandate.
The roll-out of vaccinations against Covid-19 has boosted confidence in a return to pre-pandemic life in the US.
On Tuesday, President Biden said the US was on track to have enough vaccines for every adult in the country by the end of May.
Yet the wave of reopenings has put states at odds with the Biden administration and its senior health officials, who have reacted with dismay to the relaxation of coronavirus measures at a precarious time in the pandemic.
On Monday the director of the US Centers for Disease Control and Prevention (CDC) warned of a "potential fourth surge of cases" if the country lapsed into complacency.
Covid-19 data shows that, while infections and deaths have declined in recent weeks, they are still at high levels relative to other countries.
In total, the US has recorded more than 28 million infections and 516,000 deaths related to Covid-19, according to data collated by Johns Hopkins University.
https://www.bbc.com/news/world-us-canada-56255701
By Sangmi Cha
A 63-year-old nursing home patient with cerebrovascular disease, developed symptoms including high fever, after being given the vaccine four days ago, Korea Disease Control and Prevention Agency (KDCA) Director Jeong Eun-kyeong told a briefing.
The man was moved to a larger hospital on Tuesday, but died after showing symptoms of blood poisoning and pneumonia, Yonhap news agency reported.
Another nursing home patient in his 50s with a cardiac disorder and diabetes died on Wednesday after suffering multiple heart attacks, having received the vaccine a day earlier, the agency said.
KDCA said it is investigating the cause of the deaths, but did not confirm any causal relationship to the vaccine. The agency earlier said it will provide compensation of over 430 million won ($383,466) for deaths from the COVID-19 vaccine.
“KDCA is conducting epidemiological surveys with relevant local authorities... to confirm any link with inoculation,” said Jeong.
An AstraZeneca spokeswoman in Seoul said the company had no comment.
Jeong noted that there were no cases of fatalities from receiving COVID-19 vaccines developed by AstraZeneca or Pfizer/BioNTech. However, did urge people to take the shot when they are feeling in good health.
The KDCA said that out of the people who had received the coronavirus vaccines, 207 had adverse reactions, including three cases of severe allergic reactions, known as anaphylaxis.
South Korea began vaccinating its population last week. By Tuesday midnight, 85,904 people had received the first doses of the AstraZeneca vaccine and 1,524 had been given Pfizer shots, KDCA said in a statement.
South Korea reported 444 new confirmed COVID-19 cases on Tuesday, up from 344 on Monday, raising the country’s tally to 90,816 infections, with 1,612 deaths.
https://www.reuters.com/article/us-health-coronavirus-southkorea/south-korea-probes-deaths-of-two-who-received-astrazeneca-covid-19-vaccine-idUSKCN2AV0D5
By Reuters Staff
NEW DELHI (Reuters) - Government ministers and officials were following Prime Minister Narendra Modi lead by opting on Tuesday for an Indian-made COVID-19 vaccine approved without late-stage efficacy data, instead of the AstraZeneca one.
India’s health, foreign and law ministers, and state governors, all flocked to Twitter to express support for the much-criticised Bharat Biotech’s COVAXIN vaccine, after it was administered to Modi on Monday.
“Made-in-India vaccines are 100% safe,” Health Minister Harsh Vardhan said after being inoculated with COVAXIN.
Many state officials and doctors have refused to take COVAXIN before its effectiveness could be proved. Bharat Biotech says it has completed the late-stage trial and results will be out this month.
The company said the endorsement by Modi and other ministers would set an example for other Indians and reduce “vaccine hesitancy”. It is seeking to sell COVAXIN to countries including Brazil and the Philippines.
COVAXIN and the AstraZeneca vaccines were approved by India's regulator in January. The government has distributed to states a total of 50 million doses of the vaccines but only 12% of the 12 million people immunised so far have taken COVAXIN, according to government data dashboard.cowin.gov.in.
https://www.reuters.com/article/us-health-coronavirus-india-vaccine/modis-ministers-choose-made-in-india-vaccine-over-astrazeneca-idUSKCN2AV0M6

Kenya's Cabinet Secretary for Health Mutahi Kagwe reacts after receiving the first batch of AstraZeneca/Oxford vaccines under the COVAX scheme against the coronavirus disease (COVID-19) at the Jomo Kenyatta international airport in Nairobi, Kenya March 3, 2021. © REUTERS/Thomas Mukoya
Millions of coronavirus shots from the global Covax scheme have arrived in Nigeria, Angola and Kenya, as African countries ramp up their vaccine rollouts.
Richer countries have surged ahead with vaccinations but many poorer countries are still awaiting deliveries, prompting the World Health Organization (WHO) to warn that the crisis cannot end unless everyone can inoculate their populations.
The Covax facility, run by the WHO along with health NGOs, is aiming to supply vaccines to dozens of countries in the first 100 days of 2021, and two billion doses by the end of the year.
While the continent's most populous country Nigeria received almost four million jabs on Tuesday, Angola received more than 600,000 doses and DR Congo was scheduled to get a consignment later, following recent deliveries to Ghana and Ivory Coast.
Kenya received its first shipment of just over 1 million Covax-funded AstraZeneca/Oxford shots early Wednesday.
However, there are still critical hurdles for the scheme's rollout in vast African countries with sketchy infrastructure and an array of security challenges -- a point addressed by Faisal Shuaib, director of Nigeria's primary healthcare agency.
"States without a functional airport will have their vaccines transported by road using vans with fitted cold cabins, from the nearest airport," he said.
He called the delivery -- which arrived around noon in the capital Abuja -- a "good day for Nigeria" and promised the rollout would begin in earnest on Friday with frontline health workers the first to be inoculated.
Nigerian official Boss Mustapha urged traditional rulers, religious leaders, civil society groups and the media to spread the message that vaccinations were needed, adding: "This is a fight for everyone."
In Angola, where some healthcare workers were vaccinated shortly after the doses were offloaded, the WHO's Djamila Cabral said the arrival of vaccines brought a "stronger hope to save lives", but warned that everyone needed to continue respecting Covid restrictions to beat the pandemic.
70 percent goal
The almost four million AstraZeneca/Oxford doses received by Nigeria, made by the Serum Institute of India, are the first of 16 million shots that Covax plans to deliver over the coming months to the country of 200 million.
The government said it hoped to vaccinate at least 70 percent of its adult population over the next two years and health officials said more than two million people had already registered for the jab online.
"As the vaccines arrive in batches due to limited supply we will inform Nigerians about who and where to receive the vaccine," Shuaib told reporters.
Nigeria has recorded 1,915 deaths from 156,017 cases since the start of the pandemic and Angola 508 deaths from 20,854 cases -- though official figures in most countries are considered to be underestimates.
A new virus variant has also been discovered in Nigeria, but researchers have not yet determined if it is more contagious or deadly than the original strain.
Last week, Ghana and Ivory Coast were the first African countries to receive vaccines from Covax, an initiative led by the Gavi vaccine alliance, WHO and the Coalition for Epidemic Preparedness Innovations (CEPI) with UNICEF as implementing partner.
Some 237 million AstraZeneca doses are to be delivered by the end of May to 142 participating economies, Covax says.
https://www.france24.com/en/africa/20210303-covax-scheme-rolls-out-millions-of-covid-19-vaccines-in-africa